| Week |
Progress |
| 1-7 |
Introduction to DPGraph, LaTex, and basic programming ettiquete. As a complete novice, this is very interesting to learn, but it also feels like I am studying three different languages at once. Slightly intimdated. |
|
| 8 |
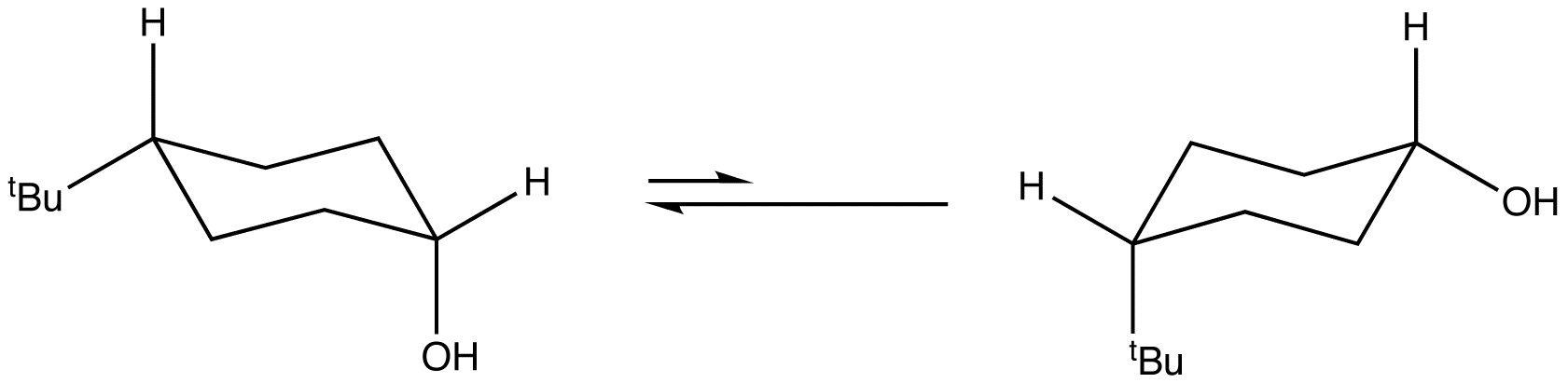
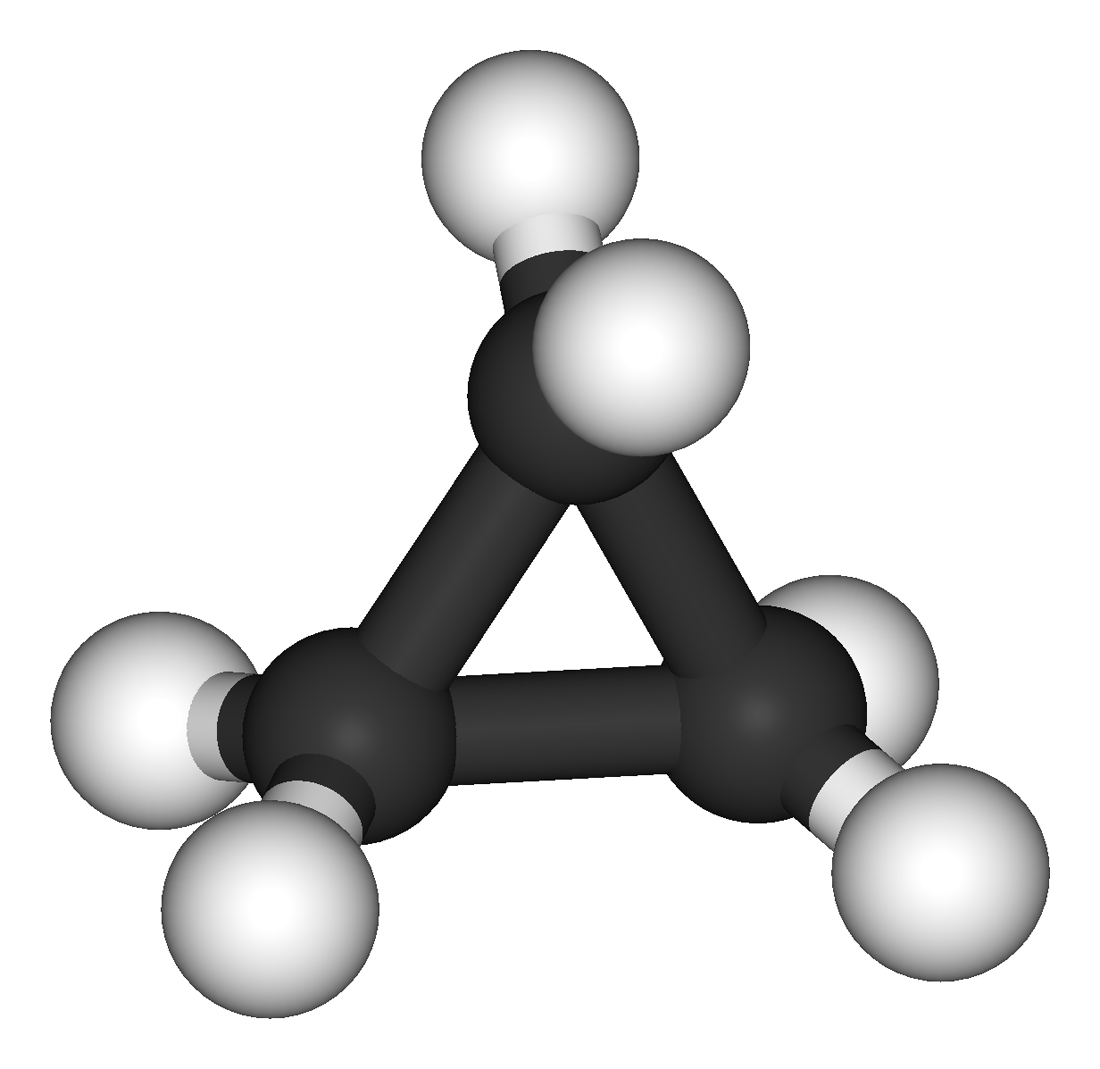
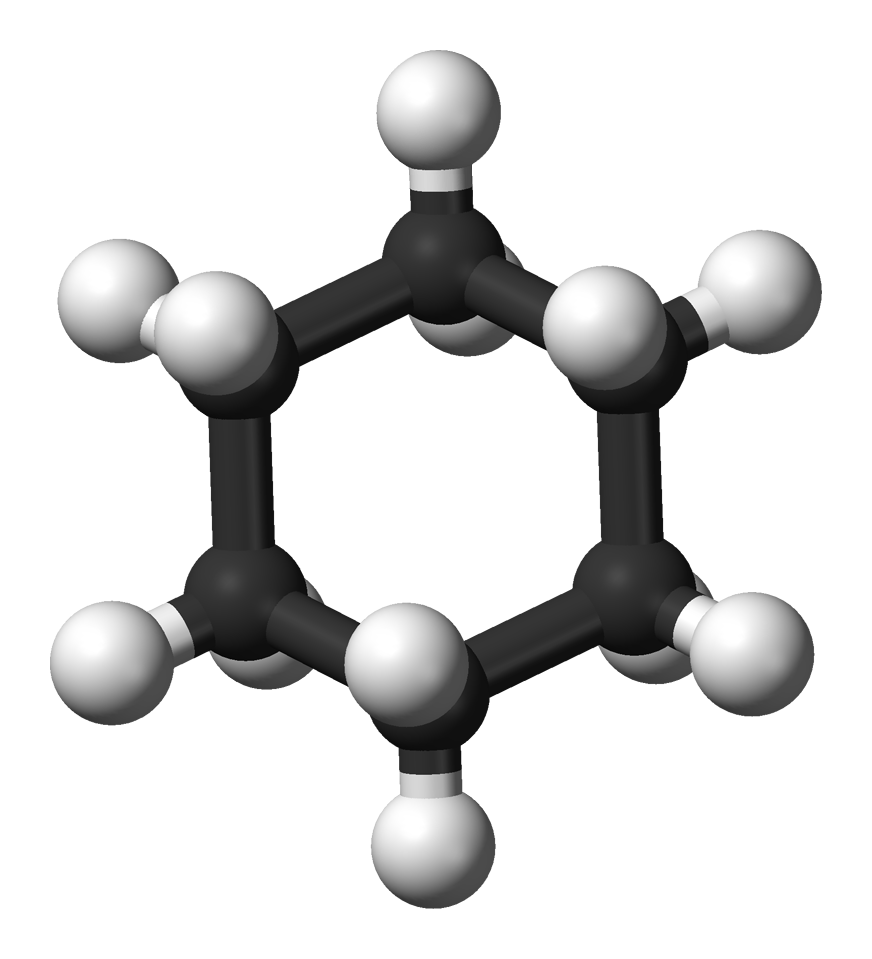
Assigned to VPython and debating between working with sound waves or some sort of chemical structure. Picked cyclohexane and chair interconversion because we are learning about it in organic chemistry and it interests me. |
|
| 9 |
Getting a feel of VPython. Language is actually very straightforward to work with, and I feel like I'm getting a deeper understanding of the program. |
| 10 |
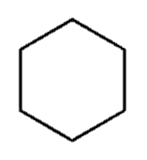
I made a cyclohexane molecule! It only took me 4 hours! |
| 11 |
Trying to figure out frames between bonds and atoms. A bit tricky, but just need to stay organized with respect to the frame and the other atoms. |
| 12 |
Figuring out frames and preliminary animations. A bit stressful. I feel behind. |
| 13-14 |
Finalized frames! Stress is almost over. |
| 15 |
Animation done! Not as hard as I thought, probably because having the frames made it a lot easier and faster. |
 .
.


 .
.