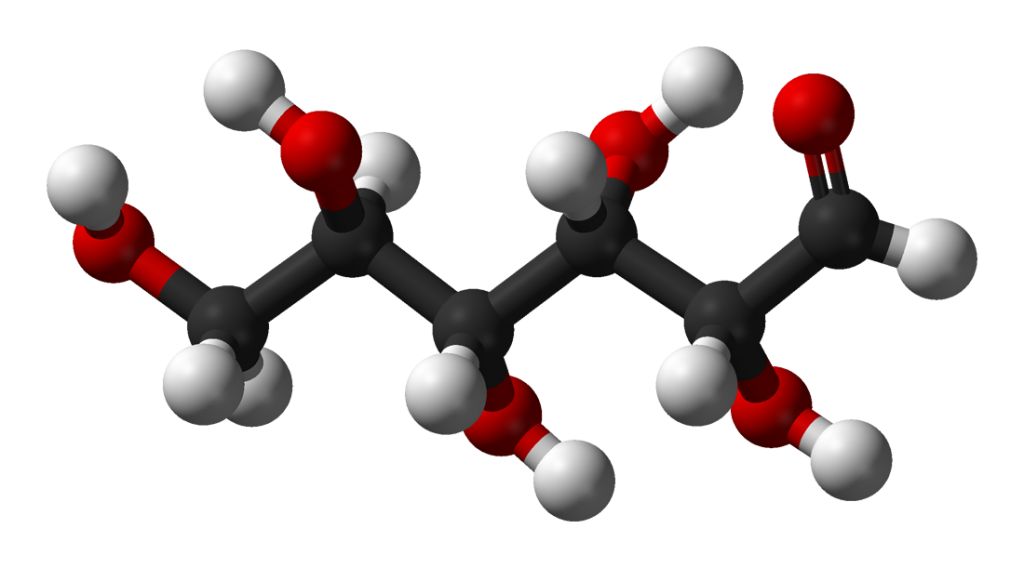

This project will demonstrate some of the molecule vibrations that cause the various results seen in a spectroscopy spectrum.
VPython was used to create the atoms and bonds and simulate their bends and stretches, which are mainly seen in infrared spectroscopy (IR).
IR spectroscopy is useful for providing qualitative information as to whether certain functional groups are present in a compound.
It is highly sensitive to changes in composition of the molecule.
However, it does not give any quantitative details. Therefore, other spectroscopy methods are used to fill in these blanks.
NMR exploits the nuclear spin of certain nuclei when placed in a magnetic field due to their magnetic moments.
Therefore, any nucleus with nuclear spin, meaning an odd number of protons and electrons, can be studied by NMR.
Some of the most commonly used atoms are proton NMR (1H) and carbon NMR (13C).
NMR is very useful when learning about the structure of a particular molecule.
However, it cannot give information on other types of atoms within the molecule besides the specific proton being analyzed,
which is why it is often used in conjuction with other types of qualitative spectroscopy.
It is also not as good as IR for identifying impurities.
More information on the math and theory behind these spectroscopy methods can be found under Documentation.
Some useful sources of information can also be found below.
Documentation
Abstract
The structure and composition of a molecule can provide useful information for chemists to learn about mechanisms and reactions.
However, it is impossible to physically see a compound.
In order to determine what the molecule is and to get an idea of its structure, scientists use various forms of spectroscopy,
which is the study of interactions between matter and light or, more generally, electromagnetic radiation.
Infrared Spectroscopy
Infrared spectroscopy (IR) utilizes infrared light to cause the bonds in a molecule to vibrate at a resonant frequency.
When they vibrate, radiation is absorbed. This absorbed radiation creates peaks on a visual spectrum.
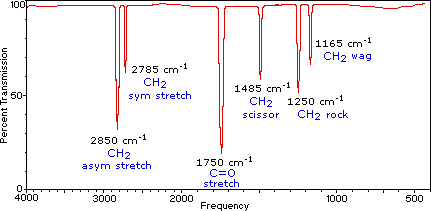
Below is a sample IR spectrum representing some various C-H vibrational modes and their corresponding wavenumbers.
 .
.
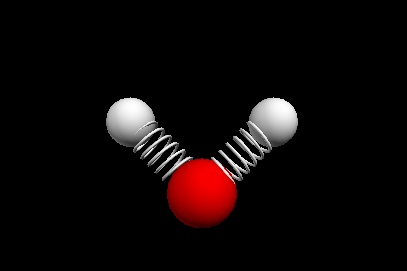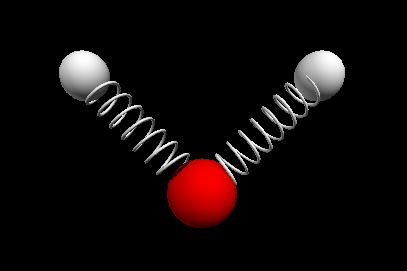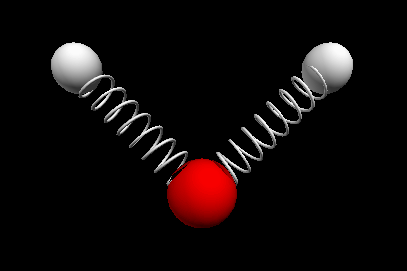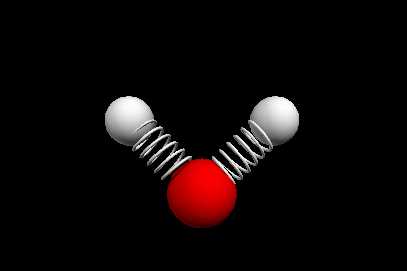
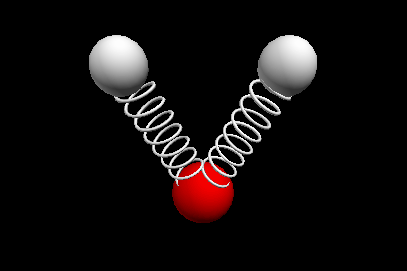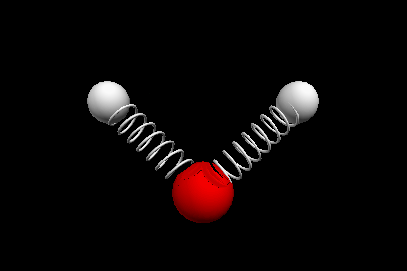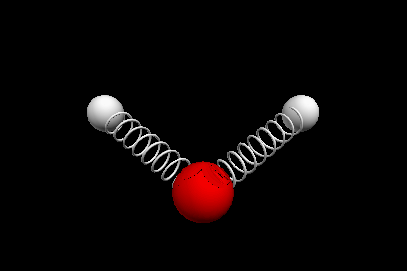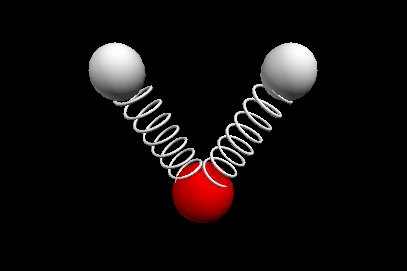
Vibrational modes of the methene or CH2 molecule:
Stretches
symmetric stretch antisymmetric stretch
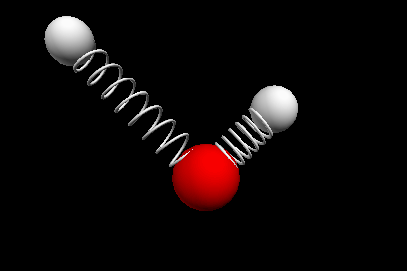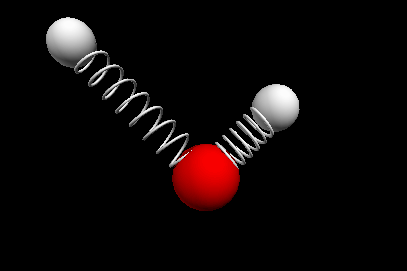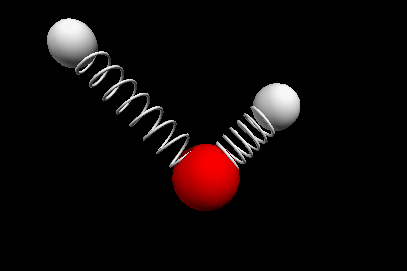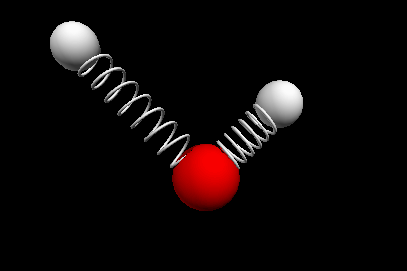
Bends
scissoring rocking
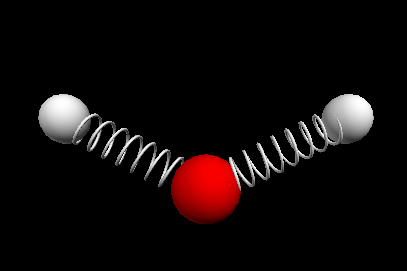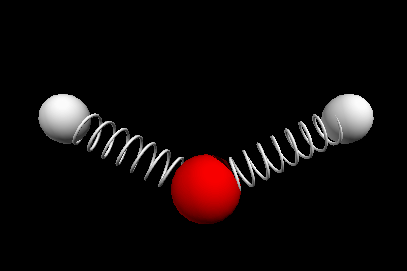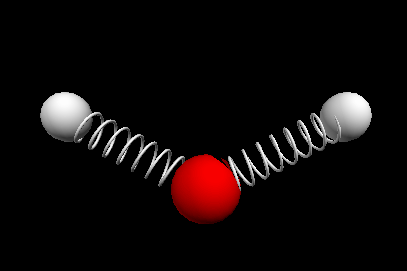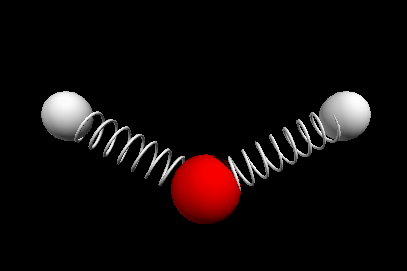
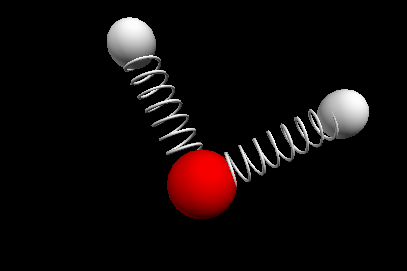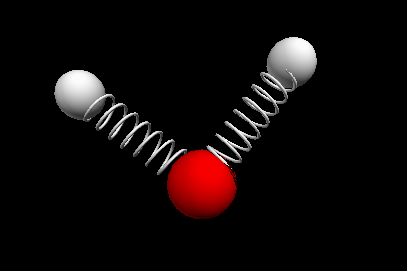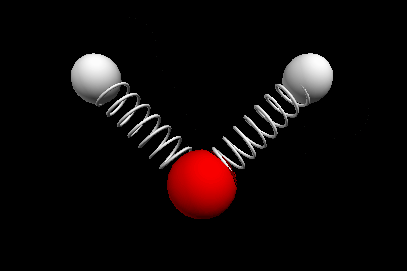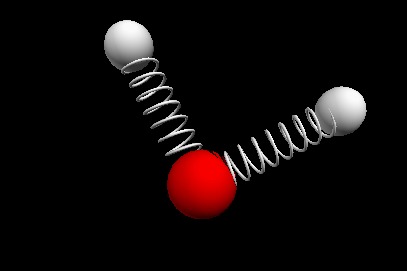
wagging twisting
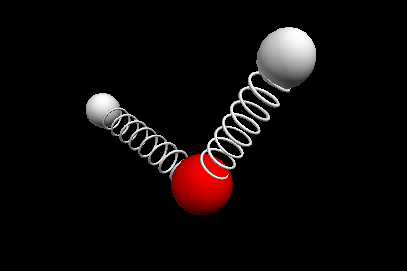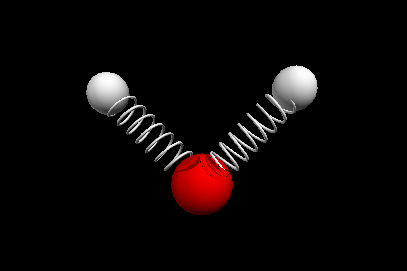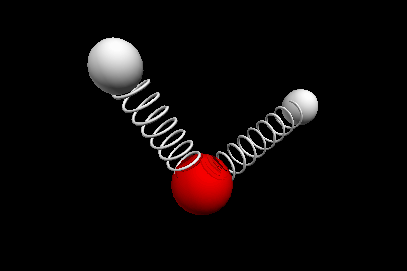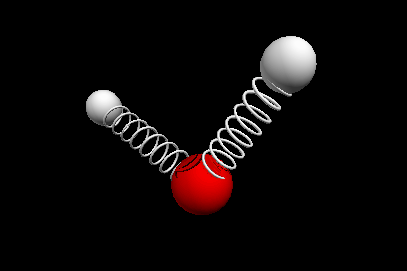
Nuclear Magnetic Resonance Spectroscopy
Nuclear magnetic resonance spectroscopy (NMR) uses radio waves, which have longer wavelengths and higher frequencies than infrared waves.
Like IR, NMR provides some qualitative information based on the location of signals in the spectrum.
However, NMR also provides valuable quantitative information to help determine a compound's structure.
Below is a sample 1H-NMR spectrum representing a small organic molecule.
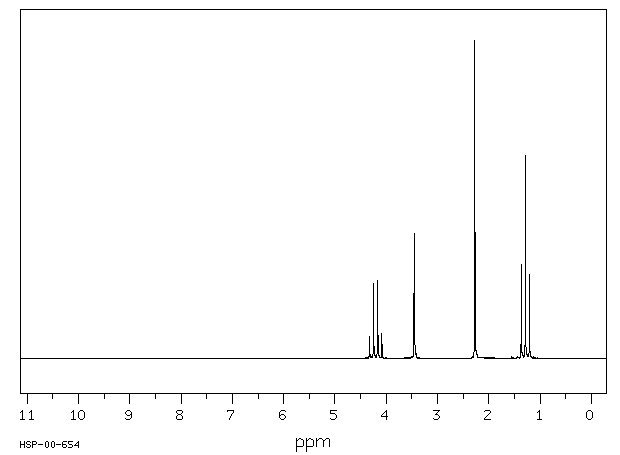
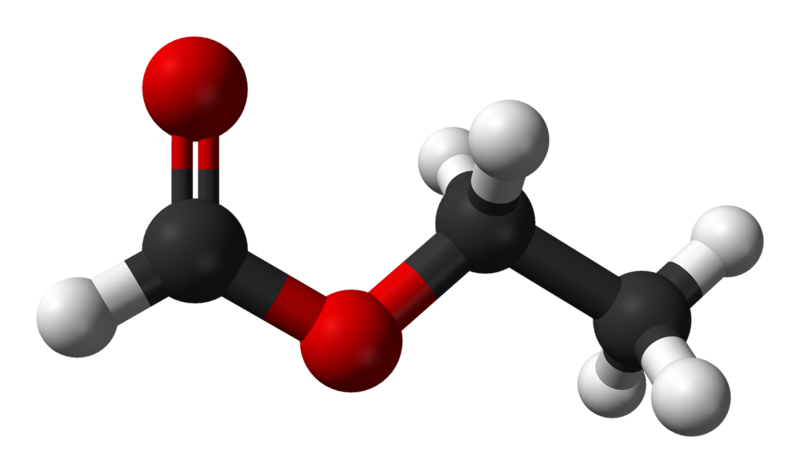 Together, both IR and NMR can be used to study both known and unknown compounds
to check for impurities and confirm a product's structure and chemical makeup.
Each has its advantages and disadvantages that contribute to drawing a final conclusion about a molecule.
Together, both IR and NMR can be used to study both known and unknown compounds
to check for impurities and confirm a product's structure and chemical makeup.
Each has its advantages and disadvantages that contribute to drawing a final conclusion about a molecule.
Sources